Metalloids, also known as semi-metals, occupy a unique position in the periodic table. They are elements that straddle the line between metals and nonmetals, possessing characteristics of both. This unique group of elements includes boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te), and polonium (Po).
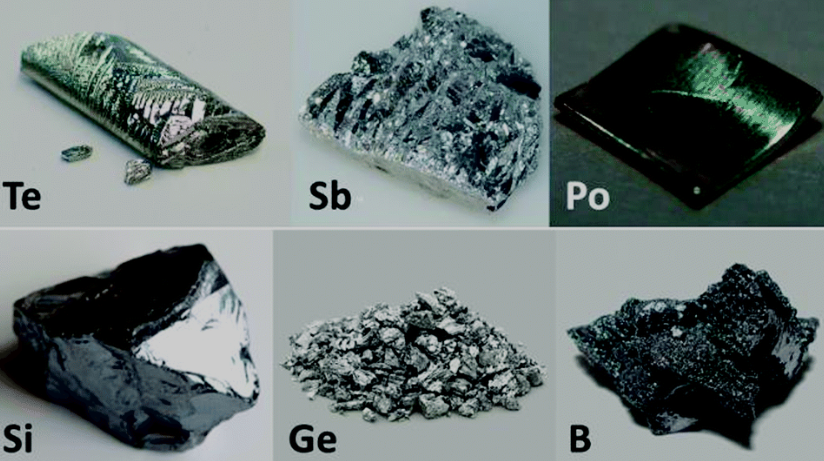
Properties of Metalloids
Though the properties of metalloids can vary greatly, there are six key characteristics that most share:
- 1. Physical Appearance
Metalloids generally have the appearance of metals. They are usually shiny, silvery-grey, and solid in their standard state. However, their appearance can also be dull, akin to nonmetals, depending on the specific element.
- 2. Brittleness
Unlike metals, which are typically malleable and ductile, metalloids are usually brittle. This means they are prone to breaking or shattering when subjected to stress or impact, much like nonmetals.
- 3. Semi-Conductive Properties
One of the most defining properties of metalloids is their ability to act as semiconductors. They have an electrical conductivity that falls between that of metals (good conductors) and nonmetals (poor conductors). This property has led to their widespread use in the semiconductor industry.
- 4. Variable Chemical Reactivity
Metalloids can be reactive or unreactive, depending on the specific element and conditions. For example, boron is relatively unreactive at room temperature, while silicon readily reacts with oxygen when heated.
- 5. Formation of Allotropes
Many metalloids can exist in multiple structural forms or allotropes. For instance, boron can form black or red allotropes, while silicon exists in crystalline and amorphous forms.
- 6. Mixed Metallic and Nonmetallic Properties
In terms of chemical behavior, metalloids exhibit a mix of metallic and nonmetallic properties. They can lose or gain electrons in reactions (a metal-like property), but they also tend to form acidic oxides (a nonmetal-like property).
Metalloid Properties List
| Element | Atomic Number | Atomic Weight | Melting Point (°C) | Boiling Point (°C) | Conductivity (S/m) |
| Boron (B) | 5 | 10.81 | 2076 | 3927 | 1.5 x 10^-3 |
| Silicon (Si) | 14 | 28.085 | 1414 | 3265 | 2.52 x 10^4 |
| Germanium (Ge) | 32 | 72.63 | 938.3 | 2833 | 2 x 10^-2 |
| Arsenic (As) | 33 | 74.922 | 817 (sublimes) | – | 3.3 x 10^-7 |
| Antimony (Sb) | 51 | 121.76 | 630.63 | 1587 | 3.1 x 10^-4 |
| Tellurium (Te) | 52 | 127.60 | 449.51 | 989.8 | 1 x 10^-7 |
| Polonium (Po) | 84 | 209 | 254 | 962 | – |
Uses of Metalloids
The unique properties of metalloids make them extremely useful in a variety of applications. Silicon, for instance, is a fundamental material in the semiconductor industry, used in the fabrication of microchips. Arsenic is used in the production of pesticides, while antimony and tellurium are used in the manufacture of flame retardants and solar panels, respectively.
| Metalloid | Key Uses |
| Boron (B) | -Boron is used in the production of borosilicate glass, which is resistant to thermal shock, making it ideal for laboratory glassware and cookware. -It’s also used in the creation of boric acid, a substance used in insecticides and antiseptics. -Boron is used in the manufacturing of semiconductors, detergents, and in neutron shielding in nuclear reactors. |
| Silicon (Si) | Silicon’s primary use is in the semiconductor industry, where it forms the base material for microchips. It’s also a key component in the production of solar panels. Additionally, silicon is used as a primary ingredient in silicone, a versatile family of synthetic materials used in sealants, adhesives, lubricants, medical applications, and more. |
| Germanium (Ge) | Germanium is used in semiconductors, especially for transistors and diodes. It’s also used in infrared optics due to its unique refractive index and in the creation of alloys. In addition, it’s used in the production of phosphors for fluorescent lamps. |
| Arsenic (As) | Arsenic is used in the production of pesticides, herbicides, and insecticides, although its use in these applications is decreasing due to its toxicity. It’s also used in the manufacturing of alloys to enhance their hardness. In the electronics industry, arsenic is used in the production of gallium arsenide, a semiconductor material used in integrated circuits and light-emitting diodes (LEDs). |
| Antimony (Sb) | Antimony is primarily used as a hardener in lead for batteries. It’s also used in the production of flame retardants and in the manufacturing of some types of glass. Additionally, antimony is used in the production of semiconductors. |
| Tellurium (Te) | Tellurium is used in the production of solar panels and semiconductors. It’s also used in the manufacturing of thermoelectric devices and as an alloying agent to improve the machinability of certain metals. |
| Polonium (Po) | Due to its high radioactivity and scarcity, polonium has limited uses. It’s used in devices like atomic batteries (used in spacecraft), and in small amounts, it was historically used in antistatic brushes. However, its use is heavily regulated due to its toxicity and radioactivity. |