Precision CNC machining has very wide applications in the manufacturing of medical components. This machining technology enables rapid production of complex medical parts, with production cycles that can be completed within a few hours. It is capable of fabricating not only surgical instruments but also critical medical devices and implants. The machining requires extremely high precision and repeatability to meet the demands of surgery, optical instruments or their maintenance and repair.

Why Precision Is Important In Medical Parts Manufacturing?
Precision is crucial in medical parts manufacturing because even the smallest deviation can compromise the safety, functionality, and effectiveness of medical devices. Components such as implants, surgical tools, and diagnostic equipment must meet strict tolerances to ensure they perform reliably in critical applications, often inside the human body.
1. Patient safety. Even minor deviations from specifications in medical parts can potentially have serious health consequences for patients. Precision ensures parts meet tight tolerances and specifications consistently to avoid any risks.
2. Proper functioning of devices and equipment. Medical devices and instruments rely on components fitting and interfacing precisely as designed. Precision is critical to ensure equipment works as intended without any performance issues.
3. Ease of assembly. Medical parts often need to be assembled with other components in complex configurations. Precision machining allows parts to fit together seamlessly during assembly.
4. Consistency. Precision CNC manufacturing provides consistency across production batches, whether producing 10 or 10,000 parts. This level of consistency is essential for parts used in medical devices.
5. Time to market. Automated precision machining simplifies and streamlines the manufacturing process, allowing medical technologies to reach patients faster through shortened development cycles.
6. Materials compatibility. Precision machining allows flexible handling of various materials like stainless steel, aluminum and plastics used in medical parts without compromising their properties.
Benefits Of CNC Machining For Medical Device Manufacturing
Precision and consistency
CNC machining allows for micron-level precision in part dimensions and geometries. Even minor deviations could compromise medical devices and implants. Tight tolerances ensure reliability and reproducible performance across batches. This level of precision protects patient safety.
Versatility in materials
CNC enables machining of bio-compatible materials like stainless steel, titanium alloys and engineering-grade plastics commonly used. Complex geometries can be achieved in a single set-up without compromising hole placement or surface finish. This gives designers more flexibility to innovate.
Cost-effectiveness for low to medium volumes
Unlike traditional tooling with high set-up costs, CNC does not require non-recurring engineering charges for production tools. It remains competitive for low to medium volumes without large minimum order quantities. This makes it suitable for customized implants and devices.
Reduced lead times
CAD/CAM integration allows quick translation of designs into machining programs. Complex parts can be produced faster from prototype to production staging compared to conventional methods. This helps rapidly fulfill urgent medical needs or customize for special cases.
Automation and reproducibility
CNC is a fully automated, digitally controlled process that consistently delivers the same high precision results batch after batch. Lights-out production is possible for higher throughput. Automation further ensures product quality and compliance with regulatory norms.
Flexibility in design changes
Program updates on CNC machines can quickly accommodate minor to medium design tweaks or refinements. This integrated process allows refining designs collaboratively based on feedback until optimal performance is achieved.
Materials Used In CNC Machining Medical Applications
| Material | Properties | Benefits | Medical Applications |
| Aluminum | Lightweight, corrosion resistant, easy to anodize for improved properties | Strong yet lightweight, provides structural support | Wheelchairs, orthopedic braces, IV stands |
| Stainless steel | Biocompatible, durable, strong, corrosion resistant, can be polished for a smooth finish | Versatile, stands up to wear and cleaning/sterilization | Bone screws, joint replacements, surgical instruments |
| Copper | Excellent antibacterial and antiviral properties | Keeps frequently touched surfaces sterile | Monitor buttons, some dental implants |
| Titanium | High strength, durable, biocompatible | Excellent replacement for stainless steel in implants & supports | Bone replacements, prosthetics, skeletal supports |
| Magnesium | High strength to weight ratio, biodegradable | Temporary bone grafts and cardiovascular stents that degrade safely | |
| Delrin (Acetal) | High strength, rigid, dimensionally stable thermoplastic | Durable yet precisely machinable for implants, tools, devices | Orthopedic implants, clamps, scalpels, inhalers, device components |
| Acrylic | Impact resistant, optically clear | Machined medical components like contact lenses, implants, dental restorations, microscope/laser parts, shields | |
| Polycarbonate (PC) | High strength, impact resistant, optically clear | Medical tubing, connectors, goggles, masks,instrument handles, device housings | |
| PEEK | Excellent strength & heat resistance for medical use | Implants, breathing tubes, clamps, X-ray/prosthetic parts resistant to sterilization chemicals | |
| PTFE (Teflon) | High heat resistance & dimensional stability | Steam-sterilizable devices&parts requiring precision &reliability like catheters, implants, prosthetics, wound dressings | |
| Polypropylene (PP) | High chemical resistance for disinfection | Test tubes, transfer pipettes, packaging, nebulizers, single-use devices like syringes & IV sets | |
| UHMW Polyethylene | Biocompatible, high strength & abrasion resistance | Bone screws, dental retainers, blades, catheters, splints, trauma plates | |
| Nylon | Versatile engineering thermoplastic | Sutures, catheters, clamps, implants, dental devices – precise yet resilient |
How To Choose Materials For Medical Device Manufacturing Using CNC Machining?
Biocompatibility
For implants that contact tissue long-term, biocompatibility is crucial. Titanium and stainless steel are preferred as they are non-toxic and compatible with the body. Plastics like PE, PU, PET and acrylics are also suitable.
Chemical resistance
Parts that undergo cleaning/sterilization need resistance to chemicals used. Stainless steel, titanium and aluminum resist most acids/bases/organics well. Plastics like PC and PEEK also perform well chemically.
Radiation exposure
Parts around MRI, X-rays or chemotherapy accumulate radiation over lifetime. Titanium withstands radiation best owing to its density. ABS, PU and PEEK are plastics used here.
Sterilization method
Materials must withstand steam, chemical or radiation sterilization without degrading. Stainless steel and titanium handle heat/repeated cleaning. PEEK, PC, PU, ABS suit steam/chemical sterilization.
Drug delivery
Aerosols/powders must not stick to distributors’ surfaces, affecting dosages. Stainless steel, aluminum, copper and titanium dissipate static well. Antistatic plastics include PP, ABS, PC and acrylics.
Joint replacements
Materials for partial/full replacements must be biocompatible, durable and wear-resistant to suit individual needs. Titanium, stainless steel, cobalt alloys and UHMWPE, PEEK, PTFE are commonly used.
Prosthetics
Design and materials consider shape-forming, strength and impact-resistance. Titanium, stainless steel and aluminum are widely used based on location/weight-bearing needs. Plastics include ABS+PC, PMMA, PEEK, glass-filled nylon, HIPS.
Standards & Systems Regarding Quality Monitoring In Medical Device Manufacturing Using CNC Machining
Quality management systems: Most medical device manufacturers follow internationally recognized quality management systems like ISO 13485, FDA’s Quality System Regulation (QSR) or MDSAP. These define procedures for designing, developing, manufacturing and servicing medical devices to consistently meet requirements for safety and performance.
Process validation: Manufacturing processes are validated to ensure any variations do not adversely impact product quality. Validation includes design reviews, installation qualification, operational qualification and performance qualification. Ongoing process monitoring also occurs.
Design controls: Rigorous design controls as per ISO 14971 are applied which includes design input, output, review, verification, validation and design transfer procedures to ensure design meets intended use and specified user needs.
traceability: Full traceability of raw materials, components and finished devices is maintained through labeling and documentation as per requirements of applicable notified bodies or regulatory agencies. This enables identification of non-conforming product if needed.
Inspections and audits: In-process and final inspections are conducted using calibrated tools and precision gaging equipment to check if parts meet technical specifications. Regulatory audits also evaluate effectiveness of quality system. Any non-conformances found are addressed and corrected.
Certifications: Medical device manufacturers obtain necessary certifications like ISO 13485, FDA registration and CE marking by demonstrating compliance to stringent quality standards and regulatory requirements through systematic auditing and inspection of facilities, processes and products.
Compliance to these rigorous quality control systems ensures safety and effectiveness of medical technologies manufactured using CNC machining and helps build customer trust. Adherence to standards is crucial in this lifesaving segment.
Specialized Medical Components That Require Precision Manufacturing
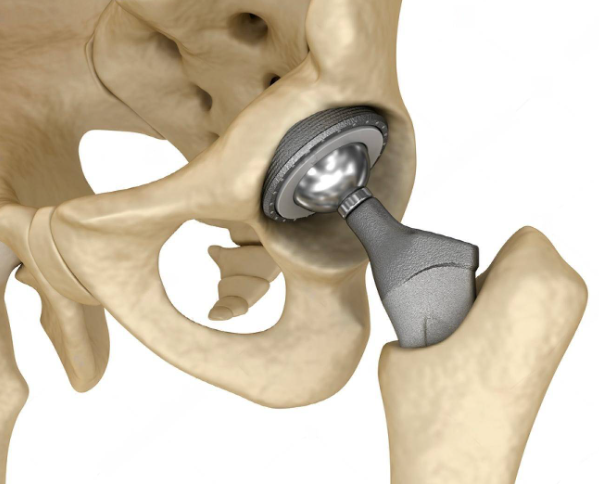
Artificial joints and implants
Implanted inside the human body, they demand high biocompatibility and precise design to properly integrate with surrounding tissues.
Surgical instruments
Such as scalpels and forceps, they need extreme strength, sharpness as well as smooth finishes to prevent wear and damage.
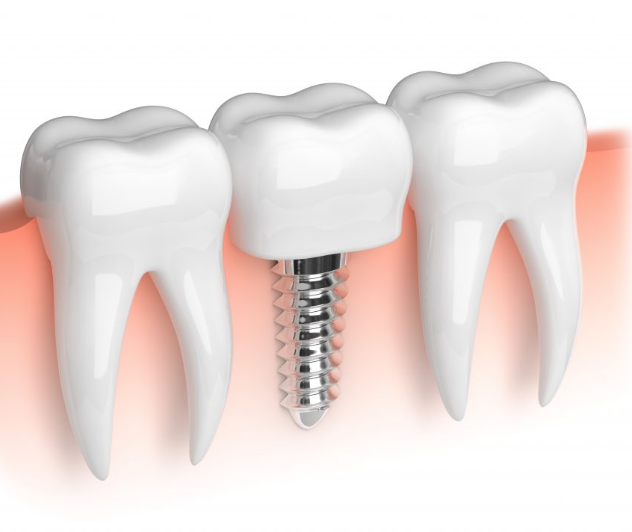
Dental materials
Prosthetics and bridges require accurate anatomically shaped designs compatible with oral cavity besides mechanical properties.
Minimally invasive surgical tools
Components like endoscope lenses and fiber optic connectors are often millimeter-sized, posing manufacturing challenges.
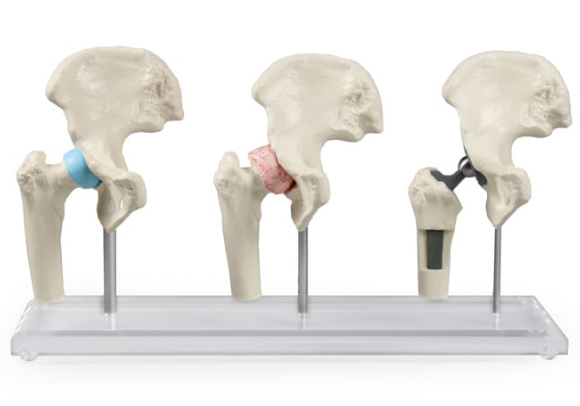
Anatomical models for implants
Complex replicated structures of spine or bones are needed to precisely restore patient’s anatomy.
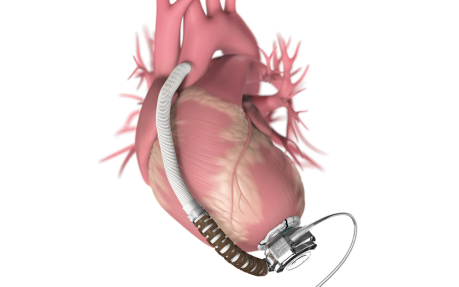
Cardiac assist devices
Parts including pacemaker electrodes ports demand high reliability and zero margin of error.